Iomab-B: Positive Phase 3 SIERRA Trial
Seeking U.S. Partner
Iomab-B is a first-in-class targeted radiotherapy intended to improve patient access to potentially curative BMT by simultaneously and rapidly depleting blood cancer, immune and bone marrow stem cells that uniquely express CD45. Multiple studies have demonstrated increased survival in patients receiving BMT, however, an overwhelming majority of patients with blood cancers do not receive BMT as current approaches do not produce a remission, which is needed to advance to BMT, or are too toxic. Studied in over 400 patients, prior studies with Iomab-B have demonstrated nearly universal access to BMT, increased survival and tolerability in multiple clinical trials including the completed Phase 3 SIERRA trial in patients with active (leukemic blasts >5%), relapsed or refractory acute myeloid leukemia (r/r AML) age 55 and above.
The SIERRA results, presented in the late-breaker session at the 2023 Tandem Meetings: Transplantation & Cellular Therapy Meetings of the ASTCT and the CIBMTR, support Iomab-B’s value proposition of enabling both improved access and improved outcomes of a BMT, thereby providing a significant curative option for r/r patients, a segment that represents approximately 50% of all AML patients and the majority not transplanted today. All patients (66/66) receiving the therapeutic dose of Iomab-B were able to access BMT with 100% engraftment. Patients in the Iomab-B arm were able to access a BMT without having to first attain a CR, and consequently were able to access BMT in approximately half the time (median of 29 days) compared to the control arm (median 66.5 days) as those patients need to attain a CR prior to BMT. 17% of patients (11/64) in the control arm were able to access a BMT per protocol analysis. Of the 77 patients on the control arm, 14 proceeded to transplant, 44 were allowed to cross over to the Iomab-B arm and 18 had no further treatment. Of the 40 crossover patients who received the therapeutic dose of Iomab-B, all 40 patients proceeded to transplant.
The primary endpoint of 6-month dCR was met with a high degree of statistical significance (p<0.0001). 75% of patients (44/59) receiving Iomab-B achieved an initial remission 30 days after their BMT compared to 6.3% of patients (4/64) in the control arm. 22% of the patients receiving Iomab-B maintained dCR lasting 180 days or more despite limited optionality for post-transplant maintenance while none of the patients on the control arm achieved dCR. The current standard practice is to administer post-transplant maintenance therapy to reduce chances of relapse.
Overall Survival for Patients who Achieved 6-month dCR with Iomab-B

Patients who achieved 6-month dCR had 92.3% 1-year survival and 59.9% 2-year survival. Median OS had not been reached in these patients. Two years in CR is a significant milestone in this patient population, highly indicative of long-term survival and a possible curative outcome.
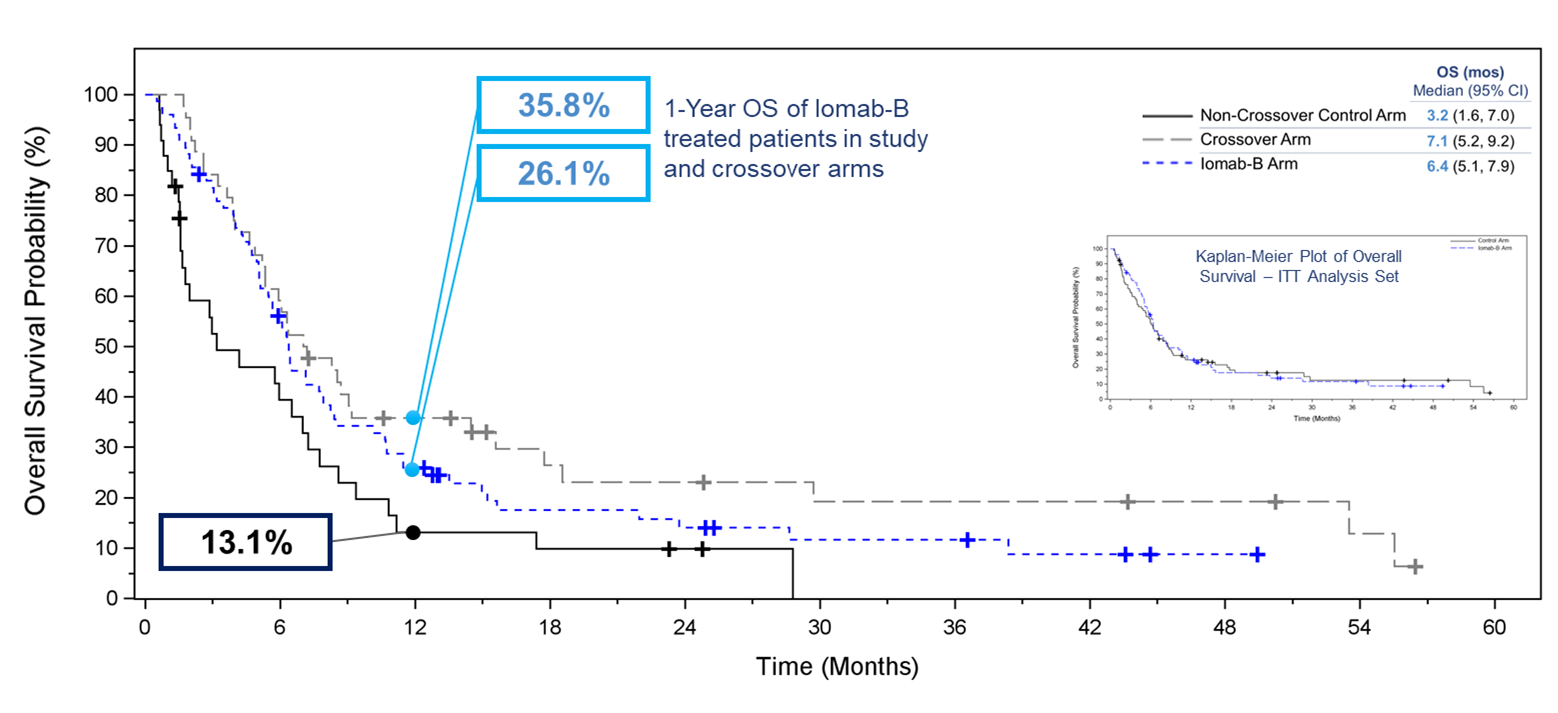
Overall survival was one of the secondary endpoints of the study. One of the exploratory efficacy endpoints was the comparison of OS in subjects randomized to the control arm who crossed over to receive Iomab-B versus all others in the control arm. Due to the crossover design, OS in the control group was confounded by the crossover and while this secondary endpoint was not met in the Intent-to-Treat (“ITT”) population, with median OS in the Iomab-B group being similar to that in the control group; after excluding crossover subjects from the ITT Analysis Set the median OS of 6.4 months for the Iomab-B group was double that of 3.2 months for the control group. A similar pattern favoring the Iomab-B group was seen across the pre-defined subgroups. Notably, the OS curves showed greater separation in favor of Iomab-B at later time points and the proportion of subjects alive at 1 year was 26.0% in the Iomab-B group compared to 13.1% in the control group.
This doubling of median and 1-year survival and a high proportion of long-term survivors in the dCR group with Iomab-B is of great clinical significance. Until recently, this group of patients had limited treatment options and while prolongation of survival was attempted, cure was never achieved. The potential of Iomab-B to double the 1-year survival and achieve cure in a subset of these patients is a significant improvement over existing treatment options.
Kaplan-Meier Plot of Overall Survival ‒ Iomab-B, Crossover, and Non-Crossover Control Arm
Iomab-B produced a significant and clinically meaningful improvement in the secondary endpoint of EFS, with a 78% reduction in the probability of an event (Hazard Ratio=0.22, p<0.0001). EFS at 180 days for the Iomab-B arm was 28% compared to 0.2% for the control arm. In the SIERRA trial, an event is defined as one of the follows: a patient not achieving CR/CRp or crossing over, patient not receiving BMT and a patient relapsing or death. The initial vertical drop in the curve in the Iomab-B arm represents those patients who did not achieve a remission after Iomab-B or those who did not proceed to transplant, while the initial vertical drop in the curve in the control arm mainly represents patients who did not achieve a remission with salvage therapy and either crossed over to Iomab-B or went onto best supportive care.
Event-Free Survival with Iomab-B Versus Control Arm

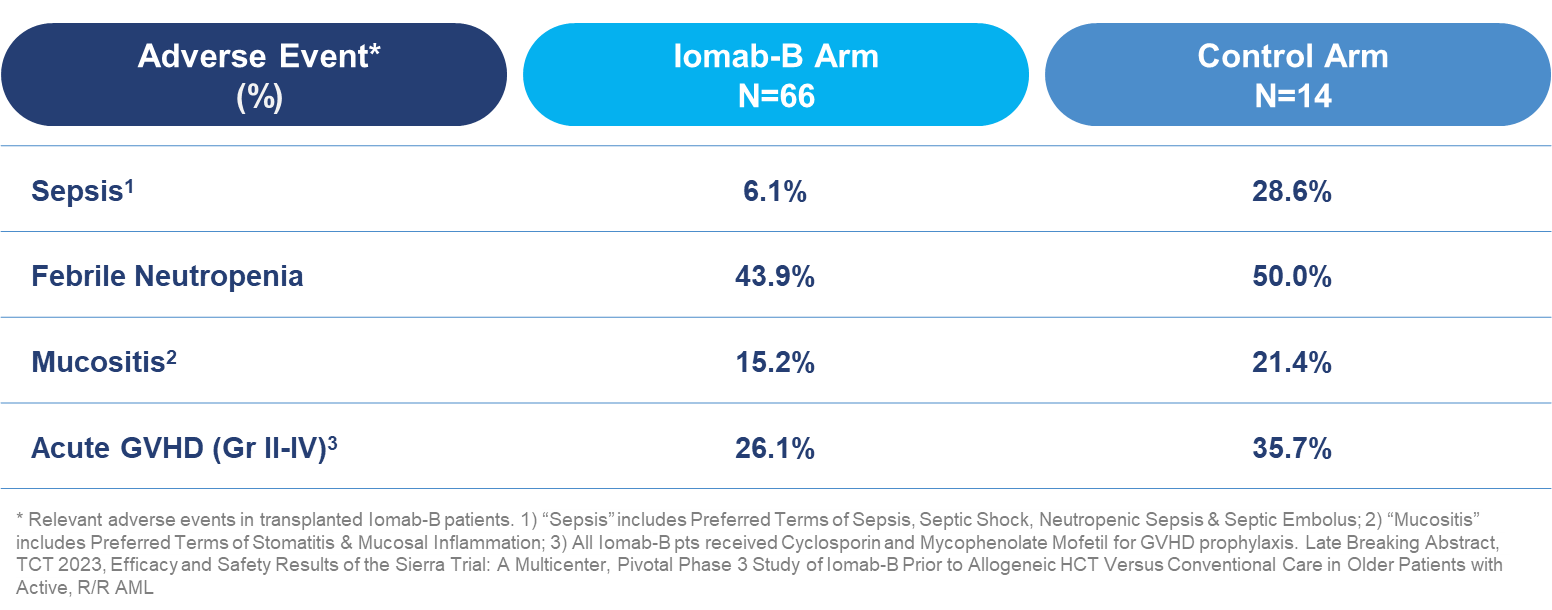
In transplanted patients, incidence of sepsis was four times lower in the Iomab-B arm than the control arm (6.1% vs. 28.6%). In addition, rates of other treatment related adverse events were lower in favor of Iomab-B, including febrile neutropenia (43.9% vs. 50.0%), mucositis (15.2% vs. 21.4%) and acute graft versus host disease (“GVHD”) (26.1% vs. 35.7%).
Grade ≥3 Treatment-Emergent Adverse Events in Transplanted Patients Through Day 100 Post-HCT
Iomab-B was developed at the Fred Hutchinson Cancer Research Center where it was studied in several Phase 1 and Phase 2 trials in almost 300 patients in multiple blood cancer indications, including acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), Hodgkin's disease (HD), Non-Hodgkin lymphomas (NHL) and multiple myeloma (MM) and is currently being studied in several ongoing physician trials. Iomab-B has been granted Orphan Drug Designation for relapsed or refractory AML in patients 55 and above by the U.S. Food and Drug Administration and the European Medicines Agency.
