ATNM-400
Next-Generation, Non-PSMA, Ac-225 Prostate Cancer Radiotherapy
ATNM-400 is a novel antibody radioconjugate designed to deliver the potent alpha-emitter Ac-225 to prostate cancer cells by targeting a non-PSMA, disease-driving protein overexpressed in advanced and treatment-resistant disease. Unlike PSMA-targeted agents that primarily serve as imaging and targeting tools, the ATNM-400 target is directly implicated in tumor progression, survival signaling, and resistance to AR inhibition. This target is markedly overexpressed in advanced, treatment-refractory prostate cancer and is associated with faster progression to castration resistance and poorer overall survival in metastatic castrate resistant prostate cancer (“mCRPC”).
We believe that ATNM-400 is uniquely positioned to overcome key limitations of current standards of care in prostate cancer, including Lu-177-PSMA-617 (Pluvicto®) and the ARPI enzalutamide (Xtandi®). By selectively targeting ARPI-resistant tumor populations and delivering potent Ac-225 alpha radiation, we believe that ATNM-400 has the potential to achieve deep, durable responses in patients with limited treatment options. With a differentiated mechanism of action, we believe that ATNM-400 represents a potential first-in-class targeted radiotherapy designed to extend survival and address the significant unmet need in mCRPC.
Preclinical Highlights
In vitro and in vivo studies have established ATNM-400’s robust therapeutic activity and unique resistance-overcoming potential:
Mechanism of Action: ATNM-400 selectively binds and internalizes in human prostate cancer cells expressing the target protein, inducing potent cytotoxicity via alpha radiation from the Ac-225 isotope, which causes irreversible double-stranded DNA breaks and targeted tumor cell death.
Sustained and specific tumor uptake: The ATNM-400 antibody showed sustained tumor uptake up to 216 hours with rapid clearance from normal tissues in prostate cancer in vivo models. PET imaging confirmed tumor-specific uptake, which was blocked with unlabeled cold antibody.
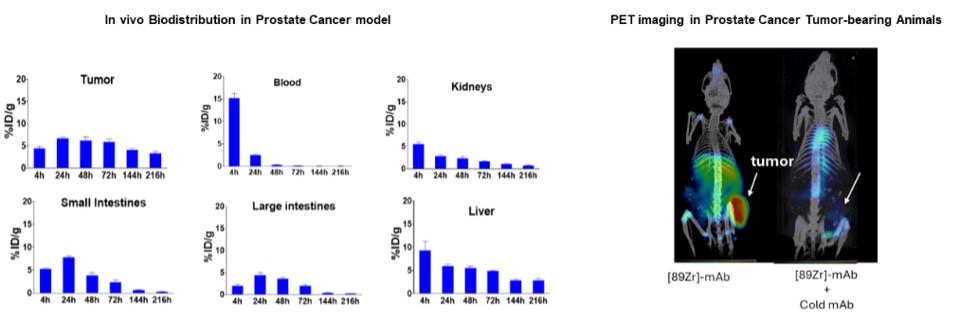
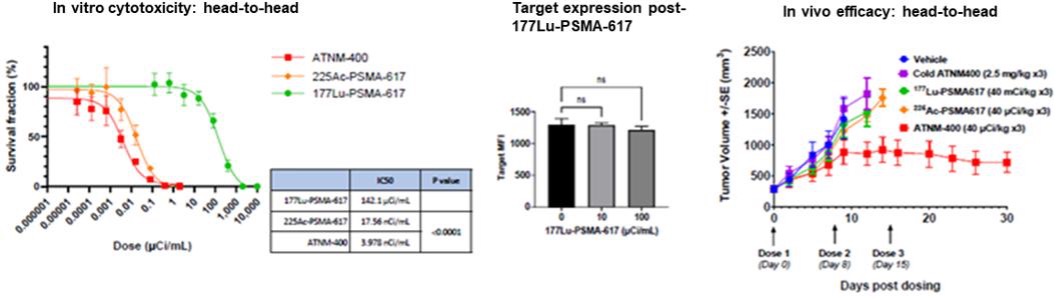
Superior to PSMA-targeted Therapies: In direct head-to-head studies, ATNM-400 demonstrated greater in vitro cytotoxicity and in vivo tumor growth inhibition compared to both Lu-177-PSMA-617 and 225Ac-PSMA-617 in prostate cancer preclinical models. Unlike PSMA, which loses target expression upon treatment with Lu-177-PSMA-617 (the active agent in Pluvicto®), the target for ATNM-400 is sustained post-Lu-177-PSMA-617 treatment.
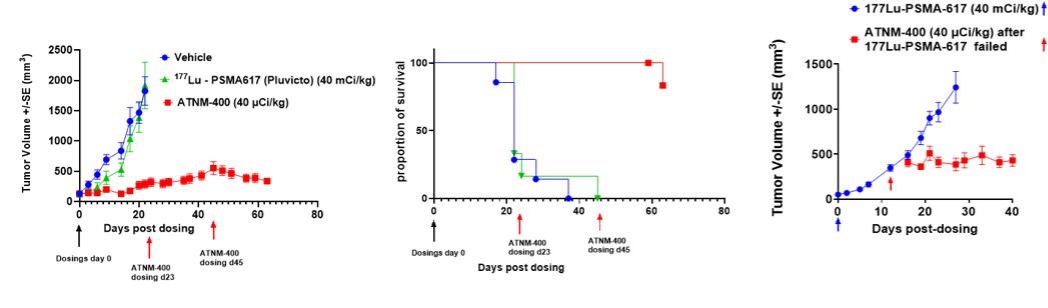
Robust Tumor Control, Improved Survival and Superior Efficacy After Lu-177-PSMA-617 Resistance: ATNM-400 outperformed Lu-177-PSMA-617, the active agent in Pluvicto®, significantly improved survival and had robust anti-tumor activity in preclinical prostate cancer models. It remained effective in both in vitro and in vivo models with acquired Lu-177-PSMA-617 resistance, highlighting its potential in resistant disease settings.
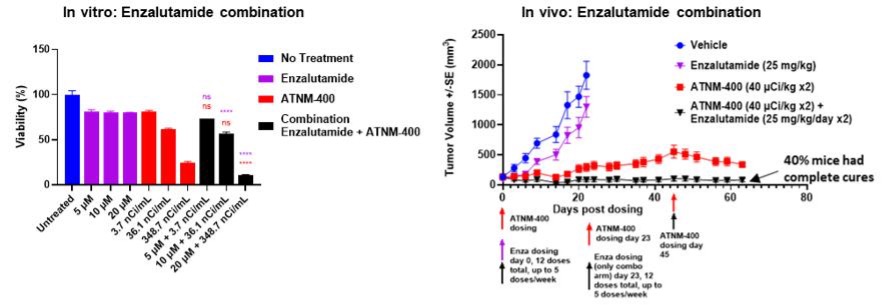
Superior Tumor Control Activity Post-Enzalutamide: ATNM-400 remained effective in preclinical prostate cancer models resistant to the ARPI enzalutamide, highlighting its utility post-AR pathway treatment failure.
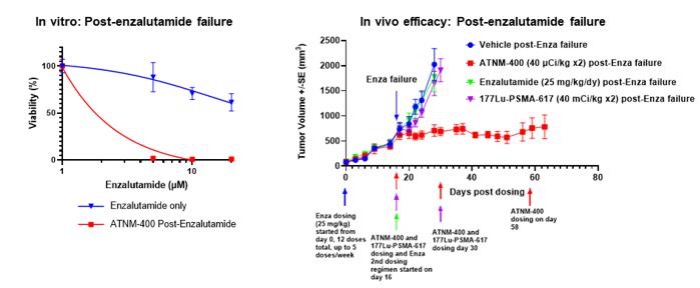
Enhanced Efficacy Supports Combination Therapy Potential: ATNM-400 demonstrated additive efficacy when used in combination with AR-targeting agents such as enzalutamide in both in vitro and in vivo preclinical studies in prostate cancer models, suggesting flexibility in sequencing or combination regimens. Also 40% of prostate cancer tumor-bearing animals had complete cures.
ATNM-400 preclinical data can be viewed here.
ATNM-400 Market Opportunity in Prostate Cancer
Approximately 313,780 men in the United States are expected to be diagnosed with prostate cancer in 2025, accounting for approximately 30% of all cancer diagnoses in men according to the American Cancer Society. Prostate cancer is by far the most diagnosed cancer in men in the United States and is the same in 118 of 185 countries. Of those diagnosed with prostate cancer, 5-7% exhibited metastatic disease at initial diagnosis, and among those exhibiting localized prostate cancer, 20-30% will progress to metastatic disease. A majority of metastatic prostate cancer patients receive hormone therapy such as ARPI therapy, as prostate cancer cells rely on androgen hormones for growth. In the U.S., approximately 40,000–60,000 mCRPC patients annually progress after ARPI therapy, which collectively generated sales of greater than $10 billion in 2024 including Xtandi® (>$5.9 billion) Erleada® (>$2.9 billion, and Nubeqa® (>$1.7 billion), highlighting a significant unmet need. Unlike localized diseased, there are no known cures for metastatic prostate cancer – there are only treatments to slow the progression of disease.
